Application process to run a study
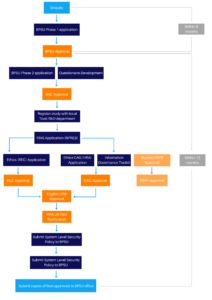
The application process to apply to conduct a surveillance study through the BPSU is outlined in this section. There are two parts to the application process, which will be thoroughly reviewed by the Scientific Committee.
Running a national surveillance study through the BPSU involves a structured application process, overseen by the BPSU Scientific Committee to ensure studies are feasible, scientifically robust, and aligned with the BPSU remit.
We now operate a two-step process that begins with early discussion and an Expression of Interest (EOI).
Step 1: Initial discussion and Expression of Interest (EOI)
All prospective study teams are asked to contact the BPSU team to discuss their research area and study concept. This allows us to provide early guidance and ensure your study aligns with BPSU criteria.
Following this discussion, teams will be asked to complete a short Expression of Interest form (Microsoft Form). The EOI will be reviewed and discussed by the BPSU Scientific Committee, who will provide initial feedback and guidance on next steps.
Step 2: Full application
If the EOI is approved, teams will be invited to submit a full application. This detailed application should include:
-
Study objectives and design
-
Proposed data collection methods
-
Patient and public involvement (PPI) plans
-
Governance and ethical approvals
A downloadable application form is available at the bottom of this page. Once submitted, the BPSU Scientific Committee will review the full application and provide feedback. Following approval and any necessary ethics or regulatory permissions, your study can begin national surveillance through the BPSU. Note: The Committee expects the submission of a full application within six months of approval of the expression of interest.
Contributions
The current contributions for running a study through the BPSU is £20,000 for the first year (13 months) and £15,000 a year for subsequent years*.